TLDR
- Eli Lilly invests $6.5B in Houston plant to make obesity pill orforglipron.
- $6.5B Houston plant boosts Eli Lilly’s obesity drug output and U.S. jobs.
- Eli Lilly expands U.S. drug production with Houston site for new obesity pill.
- Orforglipron pill at center of Lilly’s $6.5B Houston manufacturing expansion.
- Houston site fuels Eli Lilly’s GLP-1 pill ambitions with 615 new jobs coming.
Eli Lilly(LLY) closed Tuesday at $746.98, falling 1.06%, and edged down further in after-hours trading to $745.99.
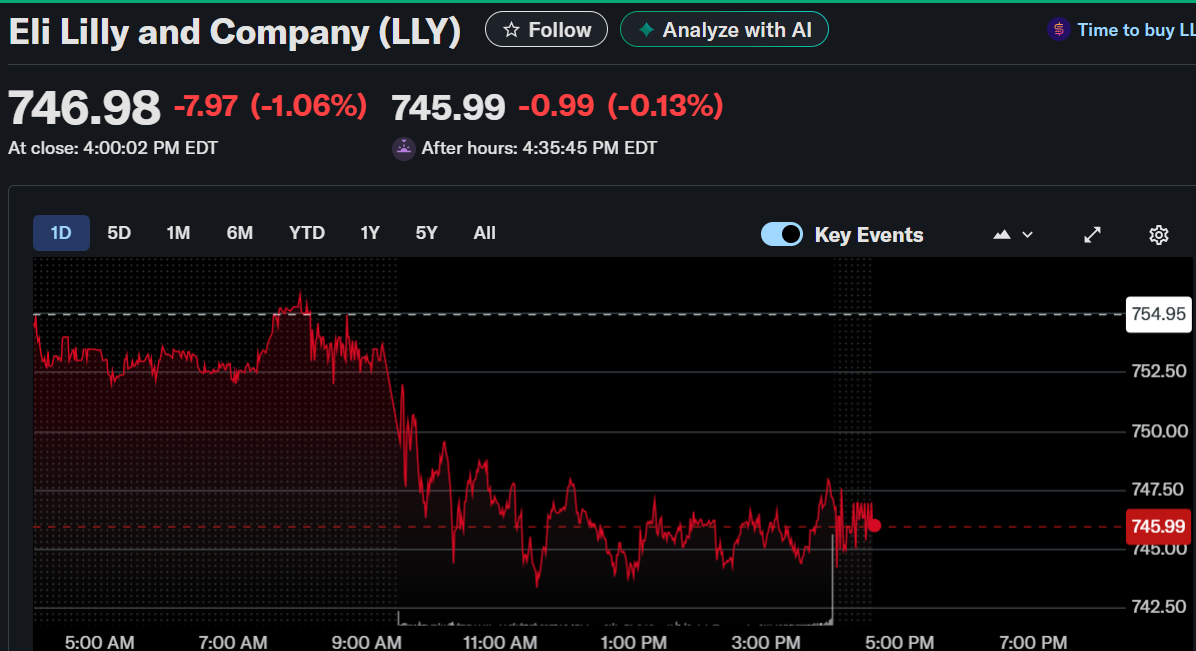
The stock responded to the company’s announcement of a $6.5 billion investment in a new manufacturing plant in Houston, Texas. This facility will support the production of its upcoming oral obesity drug, orforglipron, and other small-molecule medicines.
The Houston site represents the second phase of Eli Lilly’s planned U.S. expansion involving four major manufacturing projects. The company previously committed $27 billion in February to build these sites, adding to $23 billion in prior investments since 2020. It intends to reveal the two remaining locations later this year.
Eli Lilly aims to launch operations at all four sites within five years, enhancing output capacity to meet global treatment demand. As part of its strategy, the Houston facility will focus on advancing production of small molecule medicines. These include drugs targeting cardiometabolic conditions, cancer, immune disorders, and neurological diseases.
Obesity pill orforglipron drives manufacturing focus in Houston
The facility will primarily manufacture or forglipron, an experimental obesity pill that plays a critical role in Lilly’s future portfolio. The company seeks to capitalize on the rapid growth of GLP-1 drugs by shifting from injections to more accessible oral treatments. If approved, orforglipron will offer patients a daily pill alternative without food or water intake restrictions.
Eli Lilly continues to compete with Novo Nordisk in the GLP-1 space, where supply constraints have previously hindered growth. By investing in scaled domestic production, it aims to mitigate such risks while meeting surging market demand. The pill format also supports improved manufacturing efficiency compared to injectable formulations.
Lilly’s approach signals a strategic pivot as it ramps up preparation for regulatory approval and commercial rollout. The Houston site will play a key role in scaling manufacturing volume quickly and efficiently. This expansion supports global availability and positions the company to lead in obesity and type 2 diabetes treatment.
Job creation and political climate shape domestic drug manufacturing push
The Houston site will create 615 permanent jobs, including engineers, scientists, and lab technicians, plus 4,000 temporary construction roles. Eli Lilly emphasized the economic boost the plant brings to the Greater Houston area and its long-term employment impact. This move also aligns with broader political pressure to strengthen U.S. pharmaceutical manufacturing.
Rising concern over pharmaceutical imports and political threats of tariffs have pushed firms to prioritize domestic capabilities. Eli Lilly’s announcement follows growing federal interest in reshoring essential drug production after years of offshore reliance. The company positions this decision as both strategic and timely in today’s evolving manufacturing landscape.
While the stock declined on the announcement, the long-term expansion signals strong confidence in pipeline growth and future demand. Eli Lilly continues to bet heavily on obesity treatments and U.S. infrastructure as central pillars of its next phase. Further updates on the final two sites may bring renewed attention and shape future stock performance.